On April 6th the DEA announced it was relaxing the rules on Epidiolex, making it no longer a scheduled substance. Epidiolex, derived from marijuana, is the only cannabinoid approved by the FDA at the time.
In January, TCC published an article about how the FDA admitted during public testimony to congress that it was trying to turn marijuana into a corporate pharmaceutical concept to be pushed as prescription medications before it would consider de-scheduling the plant. The article explains how the groundwork is being laid with the CBD medication known as Epidiolex created by GW Pharmaceuticals.
The relaxing of these rules means that Epidiolex is no longer a controlled substance. It will still be a prescription-only medication but will allow doctors to prescribe it off-label and not have to be part of the DEA drug monitoring program. Before this change, doctors would have to register with a DEA drug monitoring program and would have to prescribe it primarily for some very rare forms of childhood epilepsy.
“It means doctors don’t have to go through the drug-monitoring program to make certain their patients don’t have a drug history. And a parent will now be able to go from pharmacy to pharmacy rather than having one specific source,” said William Roark, co-chair of the Pennsylvania Bar Association’s Medical Marijuana and Hemp Law Committee to the Philadelphia Inquirer.
So why the change? Why now?
GW Pharmaceuticals is seeking to extend the drug’s uses to include seizures associated with tuberous sclerosis complex. The company has stated that it has additional marijuana-derived drugs are in the making to treat spasticity associated with multiple sclerosis and spinal cord injury. GW Pharma has another marijuana-derived candidate undergoing trials for autism and schizophrenia.
As well, it can be easily imagined that the company was not getting expected sales numbers. When their product had restrictions that would dis-incentivize doctors from prescribing it, it wasn’t go to be prescribed often. Most doctors probably don’t want the government watching their every move, looking for a reason to fine them unnecessarily.
Here’s the money to be made.
The average cost of Epidiolex for a patient paying out of pocket for this medication would be $32,500 a year. That is really not a bad deal for someone needing a dosage of extract that contains purely one chemical compared to the rest of the marketplace. That’s actually pretty rare though when you look at how many people are using full-spectrum extracts or full plant use as a form of medicine. The entourage effect keeps most of this extract issue from being needed.
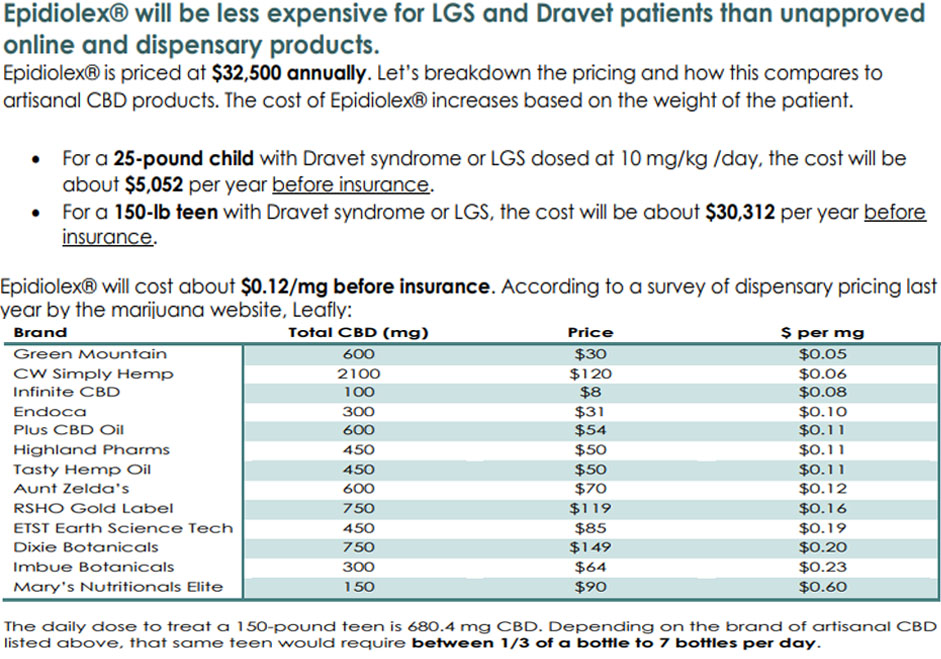
That’s where the monetary difference lies for when one starts getting into the adult needs. The price for a bottle of Epidiolex could cost between $394–$434 in a city like San Marcos, TX. That is a dosage of 100mg/1ml. The daily dose to treat a 150lb teen is 680.4 mg CBD. This puts the cost at roughly $30,312 a year or $2501/month. A 250 lb man would need 1134 mg. At $0.12/mg that puts the cost of a daily dose at $137.04. Per year that equates to $50,020 dollars. For reference, a Mercedes-Benz GLC 300 4MATIC Coupe costs $50k.
GW Pharmaceuticals can make up to $50,000 per person. But it isn’t due to restrictions by the people they’re probably lobbying, things are going to change.
Other possible impacts
An interesting thing may take place with this. The pharmacy in Austin that fills prescriptions for OTC hemp oil extract prescriptions also makes compound medications in their pharmacy. This new rule change could make it easier for pharmacies to make compound medications that will be covered by insurance. Previously the hemp oil extract was not covered by insurance. The only option was to pay cash or (flexible spending account) FSA. Being able to take insurance for people wanting a salve from CBD to put on their joints, greatly opens their ability to bring in more customers and make more money. Not just for them, but for GW pharmaceuticals as well. And that’s just one example.
Time will tell America just the reasons why this is happening the way it is. Likely, it’s not for the benefit of the consumer in the end. It may benefit the consumer, but it’s likely not the driving force.



